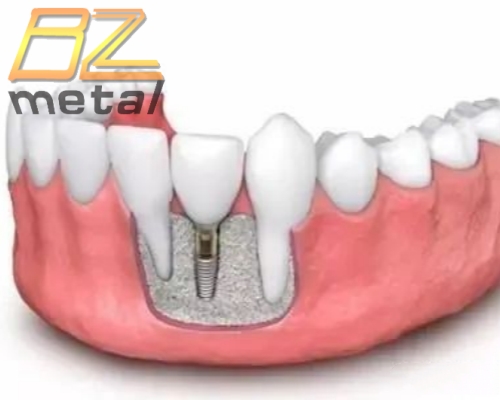
The 2nd Domestic "3D Printed Titanium Alloy Intervertebral Fusion Device” was Approved and Listed!
Big news! The 2nd Domestic "3D Printed Titanium Alloy Intervertebral Fusion Device” was Approved and Listed!
In September 2021, the National Medical Products Administration (NMPA) officially approved the listing application of Zhongnuo Hengkang's “3D-Printed Titanium Alloy Intervertebral Fusion Device”, and Zhongnuo Hengkang has become the second 3D-printed orthopedic device company in China to be approved by the NMPA for such products, 3D printing orthopedic equipment company. The product was developed and clinically verified by Zhongnuo Hengkang for six years, and completed the listing and registration approval of the innovative product with independent intellectual property rights, thereby further expanding the medical market for metal 3D printing products and promoting the commercialization of 3D-printed titanium alloy spine implants, and conducive to its clinical application in degenerative spine diseases.
Additive Manufacturing technology, also known as 3D printing technology, is based on three-dimensional digital model data as a template, and the method of manufacturing products by gradually increasing raw materials is different from the traditional material-reducing manufacturing process. Zhongnuo Hengkang uses Electron Beam Melting EBM to additive manufacture titanium alloy intervertebral fusers, which have a porous bionic bone trabecular structure, designed to imitate natural bones and help promote intervertebral fusion of the spine.

During the process of intervertebral fusion of the spine, the degenerated and collapsed intervertebral discs are removed and replaced with intervertebral fusions and bone grafts; intervertebral fusions can restore intervertebral height, provide initial stability for diseased vertebrae, provide bone grafting and fusion conditions, and improve the fusion rate. Since the human vertebral body is mainly composed of cortical bone and cancellous bone, there are obvious differences between the two in composition, pore size and tissue density. The bone density of the surface layer of the vertebral body is relatively thin, and the interior is mainly loose cancellous bone. Our 3D printing technology enables the intervertebral fusion device to have a porous structure by controlling the pore parameters, so that it has a certain elastic modulus while increasing the penetration rate of bone tissue. All these characteristics help promote bone fusion and reduce complications related to loosening of the fusion device and loss of intervertebral height.
Features and advantages of Zhongnuo Hengkang 3D printed Titanium Alloy Intervertebral fusion device
1. Macro Structure
It has good mechanical strength, corrosion resistance and biocompatibility.
By adjusting the size of the micropores, the 3D-printed titanium alloy intervertebral fusion device has an elastic modulus close to that of the vertebral cortical bone and cancellous bone.
The 3D-printed titanium alloy intervertebral fusion device conforms to the anatomical shape of the upper and lower end plates between the vertebrae, which is conducive to full contact with the upper and lower end plates, easy to place, good initial stability, and no need to worry about the displacement of the fusion device.
The surface roughness process enables this product to have micron and nanoscale surface roughness, which can maximize the mutual anchoring of bone and implant surfaces, thereby improving bone integration. Studies have shown that surface roughness has a beneficial effect on cell differentiation and proliferation.
2. Microstructure
The porosity of human cortical bone is 5-10%, while the porosity of cancellous bone is 75%-90%. The cancellous bone is highly porous and is composed of closely connected pores with diameters ranging from 300 to 1000 µm.
Current research believes that the minimum pore size that allows bone tissue to grow in is 100µm, and the critical size for capillary generation is 300-400µm. The effect of bone growth is better when the pore size is in the range of 300-1000µm. The interconnected pores in the porous titanium structure are conducive to bone growth.
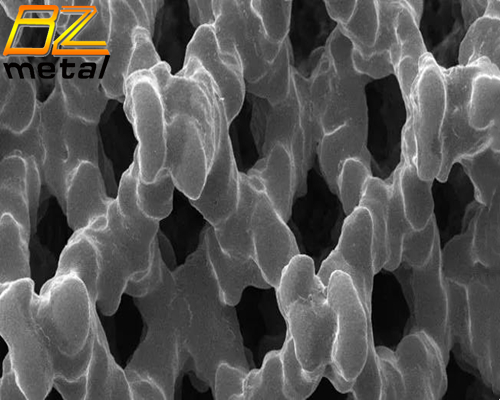
In vitro studies have shown that compared with solid titanium surfaces, human stem cells cultured on a similar porous titanium structure have a greater ability to differentiate osteoblasts.
This product has a titanium alloy bionic bone trabecular structure with a design porosity of 70-80% and a pore size of 300-800um. It can effectively provide pores for bone tissue to grow into and a surface conducive to cell adhesion, which is conducive to the growth of newborn bone through micropores into the fusion device and adhere to the metal bone trabecular surface, forming a close combination of bone and internal plants.
3. Excellent Visualization
High radiation visibility can achieve better contour display, which is convenient for clear observation of the fusion device during and after surgery, and to evaluate the contact between the vertebral body terminal plate and the implant, as well as bone fusion.
The implant is clearly visible by X-rays, there is no scattering on CT scans, MRI artifacts are reduced, and there is no scattering of tantalum markers.
4. Clinical Application
The good titanium alloy bionic bone trabecular structure design eliminates the need for bone grafting (no bone grafting window) inside the fusion device. Animal tests and clinical trials have shown that this product can make adjacent bone tissues grow freely into it, and ultimately achieve bone fusion.
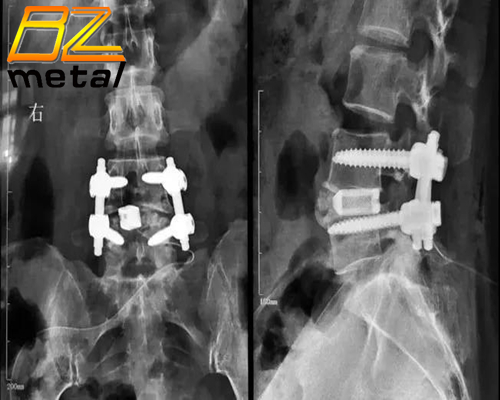
The design without bone grafting window facilitates surgical operation, reduces the possibility of contamination of the fusion device, and thereby reduces the chance of surgical infection.
This product has complete models and specifications, which can not only meet the needs of cervical and lumbar intervertebral fusion surgery, but also meet the needs of ALIF, TLIF, OLIF and other surgical methods.